Health
Addressing challenges in access to oncology medicines
|
The increasing prevalence of cancer represents a significant and growing burden worldwide. While advances in prevention, early detection, and treatment have led to improved survival, the rapid introduction of new oncology medicines has raised concerns about the ability to provide and sustain affordable access, even among the wealthiest countries around the globe. With support from the European Commission, the OECD reviewed the current state of access to oncology medicines across OECD and EU countries, and explored the policies and practices adopted by countries to address a number of challenges specific to these medicines.
|
 |
KEY FINDINGS
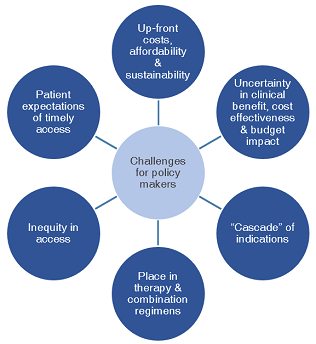
Novel oncology medicines raise a number of challenges for policy makers because of high and increasing uncertainty at the time of marketing authorisation; pricing of products with multiple indications of variable cost-effectiveness; pricing of products used in combination regimens; and budget impact driven by increasing volumes and prices of oncology medicines.
Source: Authors.
- Various strategies have been adopted to address these challenges: early dialogue between regulatory authorities, HTA institutions and companies; use of managed entry agreements to mitigate financial risks; strategies to emulate indication-based pricing for products with multiple indications; and aligning the prices of individual products used in combination regimens to the willingness to pay for that combination through confidential agreements.
- While expenditures for oncology products have steadily increased over time, budget constraints and spending caps have enabled retail pharmaceutical spending to remain stable, on average, as a share of gross domestic product (GDP) in the past decade. A few countries have established capped, earmarked budgets for the funding of innovative oncology medicines (Italy) or for the temporary funding of medicines of uncertain cost-effectiveness, pending the generation of further evidence (England and Wales).
Access to oncology medicines remains unequal across OECD and EU countries.
- The report assessed approval and coverage status across 23 countries for a sample of 109 product/indications used in five cancer sub-types and supportive care and showed wide variations (See Figure below). Access was more homogenous across countries for the subset of pairs on the WHO 21st Model List of Essential Medicines, but more heterogeneous for the subset of medicines approved in the United States since 2014.
- Marketing and coverage decisions for the 31 newer product/indications occurred at different times across OECD/EU countries, often with first approval granted in the United States. Across the sample, the average time between date of first marketing authorisation and subsequent authorisation in other countries/regions ranged from 12 to 17 months. Average total time from application of marketing authorisation in a given country/region to coverage ranged from 9 to 52 months (including clock stops).
Percentage of total sample of 109 product/indication pairs by approval and coverage status across OECD/EU countries
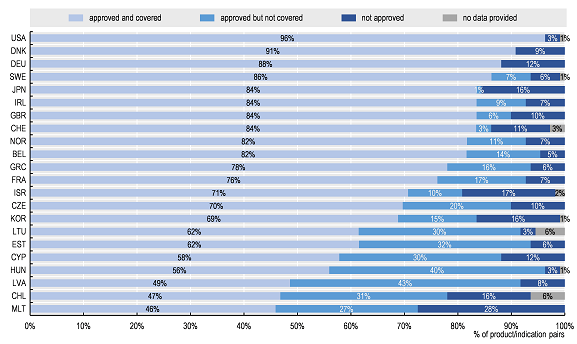
Note: Data for some countries were not included as many data were missing.
Source: Authors based on 2019 OECD survey on challenges in access to oncology medicines.
KEY RECOMMENDATIONS
Patients and clinicians are increasingly interested in international comparisons of access to medicines, and these can provide useful benchmarks for policy-makers. However, these comparisons should not be limited to simple counts of numbers of medicines approved and covered, as many other factors affect access to appropriate treatments (e.g. access to providers, levels of cost-sharing, reliability of supply chains etc.). Access needs to be understood within the context of each country's healthcare system. Where several medicines are available for a given indication, it may be possible to prioritise the use of certain medicines, based on evidence of burden of disease, clinical and cost effectiveness, without disadvantaging patients.
Countries could consider a number of policy options to address the identified challenges in access to oncology medicines:
- Enable the tracking of use by indication through routinely collected data, registries or post-marketing studies. This could serve a number of purposes, including informing ex-post price adjustments where needed, and supporting the monitoring of expenditures linked to oncology medicines, as well as contributing to 'real world' evidence of the performance of medicines.
- Improve the design of performance-based managed entry agreements to support the generation and collection of on-market evidence. This would require the collection of information on both utilisation and relevant clinical outcomes for products subject to these agreements. Harmonisation of outcome measures, data aggregation, and information-sharing across payers and countries would be highly desirable, particularly for products targeting small populations.
- Set cost-sharing arrangements, where unavoidable, as fixed co-payments rather than co-insurance, and ensure that these do not undermine access or impose catastrophic costs on households with cancer patients.
FURTHER READING
- Learn more about our work on Pharmaceuticals
- Challenges in access to oncology medicines: Policies and practices across the OECD and the EU, OECD Health Working Papers, No. 123 (November 2020)
- Addressing the challenges of access to medicines
CONTACT US
 Follow us on Twitter via @OECD_Social
Follow us on Twitter via @OECD_Social
Related Documents