Testing of chemicals
Omics technologies in chemical testing
What are Omics approaches and why are they relevant to toxicology?
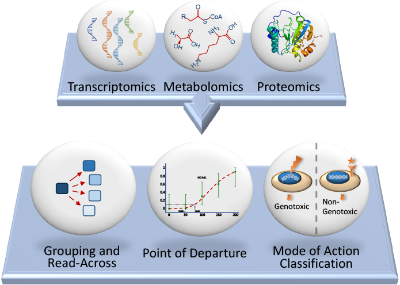 “Omics” describe approaches used in several disciplines of biology to comprehensively measure the profiles of genes (transcriptomics), proteins (proteomics), or small molecule metabolites (metabolomics) within cells or tissues. Omics approaches used in toxicology provide a tool to characterise and quantify the molecular and biochemical changes in cells, tissues and organisms following exposure to chemicals and toxic substances. Consequently, Omics are increasingly used in fields such as medicine, toxicology, and environmental science.
“Omics” describe approaches used in several disciplines of biology to comprehensively measure the profiles of genes (transcriptomics), proteins (proteomics), or small molecule metabolites (metabolomics) within cells or tissues. Omics approaches used in toxicology provide a tool to characterise and quantify the molecular and biochemical changes in cells, tissues and organisms following exposure to chemicals and toxic substances. Consequently, Omics are increasingly used in fields such as medicine, toxicology, and environmental science.
Omics measure effects across a range of biological pathways simultaneously and can be used to investigate a chemical’s mode of action, predict toxicological effects, characterise dose response relationships, and understand species relevance.
The benefits of applying omics approaches include:
- Rapid and cost-effective data generation on biological effects
- Simultaneous investigation of multiple biological pathways
- Measurement of changes to biological pathways before changes in traditional toxicological endpoints occur
- Identification of biomarkers that predict adverse outcomes
- Inform on molecular initiating events and key events in modes of action or Adverse Outcome Pathways via the AOP Wiki.
Applications of omics approaches in toxicology and risk assessment are growing, and include:
- Chemical category formation to support biological read-across
- Mode of action (MoA) analysis
- Comparison of chemical activity potencies
- Derivation of a molecular point of departure (PoD)
The OECD provides a forum for researchers and regulators to collaborate on case studies that are intended to help build consensus on the use of omics platforms in different decision-making contexts.
Why is the OECD working on Omics?
The OECD chemical safety programme provides guidance, harmonised methods and tools to help member countries implement national chemical safety policies. In this way, the OECD assists member countries in protecting human health and the environment from hazardous chemicals and making chemical management policies more efficient.
The OECD is committed to using state of the art tools to evaluate chemical safety. As a first step towards using new technologies for assessing the effects of chemicals, the data collected and reported can be standardised to all decision makers to evaluate effects and share information more easily.
One such tool is the Adverse Outcome Pathway (AOP) framework which allows the organisation of biological information on how chemicals affect biological pathways and summarises the test methods used to measure these effects. AOPs can support regulatory decision-making in numerous ways, including weight of evidence evaluation, organising information in integrated approaches to testing and assessment (IATA), predictive toxicology and more. The Extended Advisory Group on Molecular Screening and Toxicogenomics (EAGMST) has been overseeing the work on Omics, including the development of a reporting framework for transcriptomics and metabolomics until 2022. The application of Omics data in a regulatory context and the expansion of the reporting framework is now being explored by the OECD Working Party on Hazard Assessment (WPHA), in collaboration with the Advisory Group on Emerging Science in Chemicals Assessment (ESCA).
While Omics approaches have been used in toxicology research to address a range of questions, standardisation of data collection and reporting is needed to facilitate the use of omics data in regulatory decision making. A number of challenges for using Omics data in chemical risk assessment have been identified, including:
- Lack of transparency in how Omics data is generated and processed for molecular (gene, protein, metabolite, etc.) endpoints
- Lack of standardisation in how Omics study parameters and results are reported
- Lack of case studies and guidance describing acceptable (and ultimately best) practices
In response to these needs, the OECD is undertaking a number of activities to enable the translation of Omics science towards regulatory decision making, introduced below.
Current and planned OECD activities
OECD Omics Reporting Framework for Transcriptomics and Metabolomics in Regulatory Toxicology:
Increased transparency in the reporting of Omics data used in regulatory toxicology was identified as a critical priority in an expert workshop hosted by the European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC). To address this need, the OECD EAGMST launched a project to develop a reporting framework and guidance for generating and analysing Omics data. This framework was intended to aid regulatory uptake while remaining sufficiently flexible to allow technologies to evolve. As first steps, the OECD EAGMST developed a Transcriptomics Reporting Framework (TRF) and a Metabolomics Reporting Framework (MRF), and associated guidance for applying the frameworks. The Ecetoc MEtabolomics standaRds Initiative in Toxicology (MERIT) served as the starting point for the MRF. Given the highly overlapping content of the TRF and MRF, they have since been harmonised and now exist as a single, integrated, modular framework.
| Guidance Documents | Reporting templates | Date and status |
| OECD Omics Reporting Framework - Guidance document | OECD Omics Reporting Framework - Reporting template | November 2023 - Approved and declassified - For use |
| OECD Omics Reporting Framework - Reporting template | November 2022 - Draft - Not for use (Available only for versioning purposes) |
The integrated TRF and MRF were developed by international teams of experts from government agencies, regulatory bodies, industry and academia. In addition, developing reporting templates and supporting guidance for completing templates, the experts also tested the frameworks in a variety of scenarios.
More information can be found in the publication: Progress towards an OECD reporting framework for transcriptomics and metabolomics in regulatory toxicology.
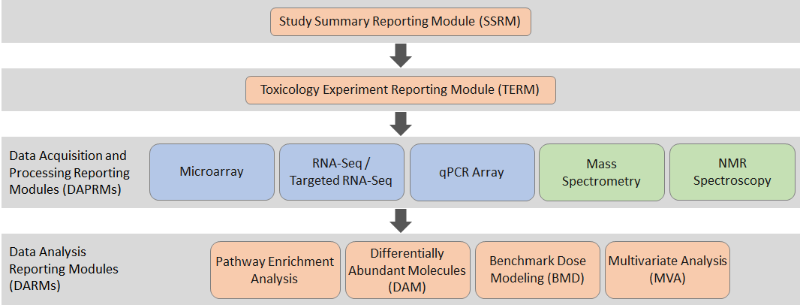
Figure: Modular structure of the omics reporting framework
The four principal types of modules for reporting transcriptomics and metabolomics studies: (1) the Study Summary Reporting Module (SSRM) to provide a high level overview of the whole study; (2) the Toxicology Experiment Reporting Module (TERM) to describe the in vivo or in vitro toxicology study; (3) Data Acquisition and Processing Reporting Modules (DAPRM) that detail the omics assays, data acquisition and processing; and (4) Data Analysis Reporting Modules (DARM) describing the statistical analysis of the Omics data. Orange modules are harmonised across the transcriptomics and metabolomics, blue modules are specific to transcriptomics, and green modules are specific to metabolomics.
Source: Harrill et al.
The framework consists of four types of reporting modules:
- The Study Summary Reporting Module (SSRM) provides a high-level overview of the regulatory toxicology and Omics experiment
- The Toxicology Experiment Reporting Module (TERM) reports the key descriptors of the in vivo or in vitro toxicology study
- Data Acquisition and Processing Reporting Modules (DAPRM) report descriptions of the Omics assays, data acquisition and associated data
- Data Analysis Reporting Modules (DARM) describe the statistical analysis that has been undertaken in the Omics study. Guidance documents describe each of the reporting modules in detail.
OECD ESCA-WPHA joint activities:
To accelerate the application of Omics in regulatory decision-making, the OECD has established an Omics Expert Group that supports WPHA’s projects on Omics and collaborates closely with ESCA. The Expert Group is examining the use and value-added of Omics data in regulatory examples and expands the OORF to cover additional technologies and data analysis modules. With this dialogue, the OECD aims to capture the needs of regulators and reflect these in the frameworks and associated Omics guidance.
Engage with us
- Stay tuned on Twitter: @OECD_ENV
- For more information, please contact: ehs.contact@oecd.org
- To receive our latest news, publications and events, sign up to the Chemical Safety and Biotechnology Update newsletters.
Related Documents